Abstract
Clinical trials have shown venetoclax (VEN) has a positive impact on health-related quality of life (HRQoL) in patients with relapsed/refractory (R/R) CLL, as well as being an effective and well tolerated intervention (Cochrane T, et al. Leukemia & Lymphoma 2022; Weirda W, et al. Presented at EHA 2017).Currently, real-world data describing the impact of VEN on HRQoL in patients with R/R CLL are lacking. This abstract focuses on the HRQoL data from the DEVOTE study, a Canadian multicenter, open label, single arm, 24-week observational study which examined the real-world characteristics of patients with R/R CLL who initiated VEN treatment.
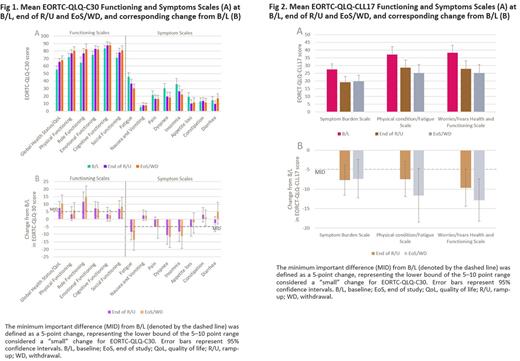
Eligible patients were >18 years old, provided written informed consent, had CLL and had received ≥1 prior line of therapy, and whose physician had decided to prescribe VEN (± rituximab) as routine clinical care. Those participating in an interventional study were excluded. The HRQoL assessments were performed at baseline (B/L), the end of the VEN ramp-up phase (R/U), and at the end of observation period, either end of study (EoS) visit at 24 weeks or early discontinuation (WD) visit. Assessments included the EORTC-QLQ-C30, a 30-item cancer questionnaire with 5 functional scales (cognitive, social, physical, emotional, and role functioning), a global health status/QoL scale, 3 symptom scales (fatigue, nausea and vomiting, and pain) and 6 single items (insomnia, appetite loss, constipation, diarrhea, dyspnea, and financial difficulties), and the EORTC QLQ-CLL17, a 17-item module specific to CLL consisting of three multi-item scales (symptom burden, physical condition/fatigue, and worries/fears health and functioning). Scores for both questionnaires, and their functional/symptoms scales, range from 0-100. For functional scales, a higher score represents an improvement in HRQoL while for symptom scales, a higher score denotes more intense symptoms. The minimum important difference (MID) from B/L was defined for both questionnaires as a 5-point change, the lower bound of the 5-10 point range considered a "small" change for EORTC-QLQ-C30 (Osoba D, et al. J Clin Oncol 1998; Osoba D, et.al. Qual Life Res 1994).
Overall, 89 patients were enrolled in the DEVOTE study and received ≥1 dose of VEN. Median (range) age was 73.0 (39-91) years, 67 (75.3%) were male and 78 (87.6%) were Caucasian. Patients had undergone a median (range) of 2 (0-7) prior lines of therapy with 79.8% previously treated with ibrutinib. Eighty-one completed R/U and 74 reached EoS. Questionnaire completion rates were high for both the EORTC-QLQ-C30 (86/89 at baseline, 66/81 at R/U and 60/79 at EoS/WD) and EORTC-QLQ-CLL17 (86/89 at baseline, 67/81 at R/U and 62/79 at EoS/WD).
Baseline EORTC-QLQ-C30 scores were aligned with previous reports for a pre-treated CLL population (Holzner B, et al. Eur J Haematol 2004). Treatment with VEN was associated with improvements in HRQoL overall, across all five EORTC-QLQ-C30 functioning scales and most of the symptom scales, which were sustained from the end of R/U to the EoS/WD (Fig 1A). Mean improvement in scores surpassed the MID of 5 for all functioning scales other than cognitive functioning, and the fatigue, pain, dyspnea and insomnia symptom scales. The nausea and vomiting and the constipation symptom scores both worsened marginally, and the diarrhea score was variable (Fig 1B). Similar results were observed for all 3 EORCT-QLQ-CLL17 domains (Fig 2A and B).
This pre-treated population showed a rapid improvement in HRQoL following initiation of VEN both in terms of functional and symptom scales, with most improvements sustained from the end of R/U until EoS/WD. There was no negative impact on HRQoL observed in association with the VEN ramp-up period. These real-world results corroborate the findings from clinical trials and the high response rates suggest observational studies are an effective forum for assessing the impact of interventions on HRQoL.
Disclosures
Aw:AbbVie: Consultancy; AstraZeneca: Consultancy. Banerji:AbbVie: Consultancy, Research Funding; AstraZeneca: Consultancy; CIHR: Research Funding; Lundbeck: Consultancy; CancerCare Manitoba: Research Funding; Gilead: Consultancy; Janssen: Consultancy, Research Funding; Roche: Consultancy; Research Manitoba: Research Funding. Bull:AbbVie Corporation: Current Employment. Fournier:AbbVie: Current Employment. Peters:BeiGene: Consultancy; Novartis: Consultancy; Incyte: Consultancy; Seattle Genetics: Consultancy; Incyte: Consultancy; Roche: Consultancy; Janssen: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy, Honoraria; Gilead: Consultancy. Owen:Incyte: Honoraria; GIlead: Honoraria; Roche: Honoraria; Merck: Honoraria; AstraZeneca: Honoraria; Janssen: Honoraria; Novartis: Honoraria; AbbVie: Honoraria; BeiGene: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.